Why Should You Test Your Body PH?
Why should you test your body pH? PH testing helps you to know how well or not well you are managing your body's pH and cell/organ health. There is an exact pH necessary for the health of blood, cells, organs, external fluids, skin and hair.
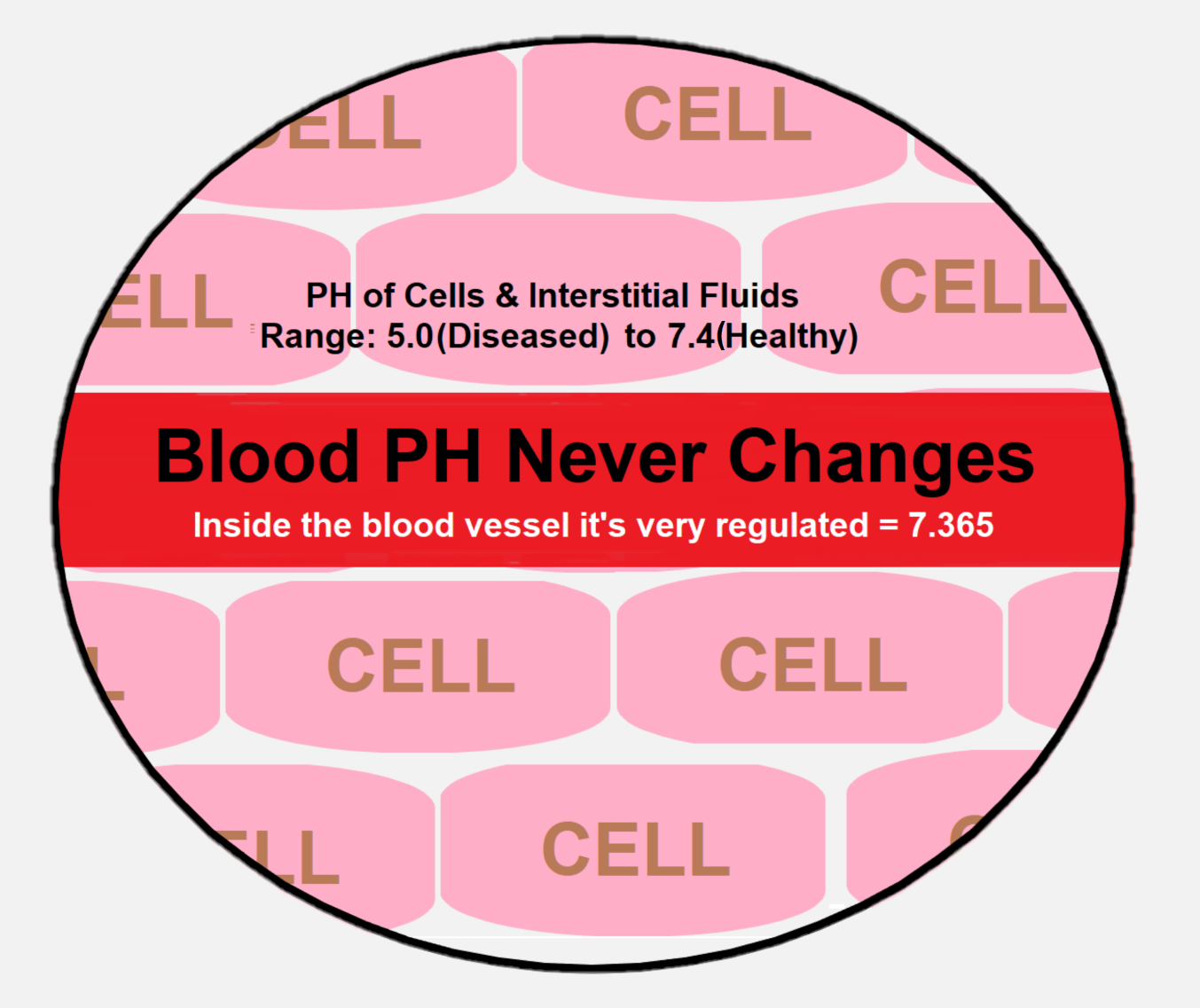
Blood pH never changes. It's always 7.365 no matter what [or you die], but the pH of cells, organs, and external fluids do change. They are typically the dumping grounds for acids that your blood cannot hold. Your body becomes diseased to the extent that you do not maintain a pH-balance in your cells, organs and external fluids.
This page is about testing the saliva and urine pH, which will help you get a good idea of the pH of your "external fluids"
Testing Body PH
Saliva pH is easy to test and is very closely aligned to the pH of blood and interstitial fluids [fluids around the cells]. The saliva pH test will reveal how well your diet, exercise and life, in general, is geared to a naturally balanced body pH. If your body is low on buffers it will rob them from joints, bones, and some of your bodily fluids, such as saliva. So your saliva pH test will come up low, signaling a warning that you need to either provide more buffers or consume less acidic foods/drinks. The urine pH test is similar, but reveals more about how well your kidneys and other organs are working to buffer your body pH by eliminating excess acidic minerals. It can clue you into the warning signs of too much stress being put on your vital organs as well. Both tests are described, below, along with the reasons why pH self-monitoring is important. And, when you do the math [see below], it costs about 2 cents per pH test and takes maybe 10 seconds to do.
How to Test Body PH
There is a very simple way to test your body pH. You can easily and inexpensively test both your pH and saliva with these simple steps. The scientifically calibrated pH paper is relatively inexpensive and well worth it to purchase.
Here's how to test saliva pH
The goal would be for your saliva pH to be somewhere around 6.8 to 7.2 [The science on this is not perfect]. I have found the results to be better when I test first thing in the morning, before consuming anything.
1. Get a white plastic spoon or plate.
2. Wiggle tongue around in mouth to stimulate saliva. When you get a good amount, about a tablespoon's-worth, then waist that [just spit it in the sink]. Then do that again and this time spit into the spoon or plate.
3. Using scissors, cut about 1/4 inch of the pH paper. And put this in the saliva.
4. Match the color with the chart on the pH paper container.
Watch this video to see a demo of testing saliva pH:
This is another quick video demo of me taking my own saliva pH
Here's how to test urine pH
The goal would be somewhere around 6.8 for urine pH. For urine, you would pee in a cup and using a tweezers dip the 1/4 inch piece of paper for about a second. Match the color to the chart as in the saliva test.
How to Regulate Body PH
If you don't consume enough alkalizing foods and at the same time consume too many acidic foods your cells will tend to retain the acid waste products plus your body will try to compensate for the deficiency by robbing your bones and joints of calcium! Most people eat about 80% meat, dairy, and grains/starches and 20% salads and other vegetables. The top nutritional experts in the field of balancing the body pH advise the opposite: 80% salads and other vegetables and 20% meat, dairy and grains. The point with this article is that any measures you take to eat more of alkalizing foods and less of acidifying foods will be an improvement. On top of that I have found for myself that drinking several glasses of ionized water every day gives me the ability to have a perfect body pH even though I am not very perfect at eating alkalizing foods all the time. I personally follow more of a 50%/50% ratio of alkalizing vs. acidic foods in my diet, but I drink over a gallon of ionized water daily to make up for it.
Alkalizing Foods to Eat More Of
There are quite a few foods that will help to alkalize your body. Most raw vegetables (especially green ones) and many fruits will, along with cold pressed olive oil, sesame and safflower oils, raw almonds, seeds, and of course ionized water. Cooked vegetables and fruits on the other hand are not as good.
Acidifying Foods to Limit or Avoid: Foods that contribute to body acidity are: meat, corn, corn products, dairy, soft drinks, coffee, Gatoraid and other drink-aids, grains, starches, and of course most "junk food".
What Happens to Your Cells if the Acid Wastes Can't be Eliminated?
Recently I've been experimenting with storing apple slices in alkaline ionized water verses tap water and acidic water. I wanted to simulate what may be happening inside our bodies when the acidic wastes get trapped in the cells. The following are some videos showing this testing. When stored in ionized water the apple slices maintained their good taste and firm texture. When stored in tap water, they maintained their firm texture, but tasted bitter. When stored in acidic water, the apple slices rotted. Watch the videos:
The Real Reason to Test your pH is that the Body pH and Blood pH are Indicators of Health
To stay healthy, your body pH must be maintained in the slightly alkaline range. A body that tends toward acidity heightens the risk for infections from bacteria, yeast, parasites, and viruses. All of these seek out and thrive in an acid environment. Virtually all degenerative diseases including heart disease, arthritis, osteoporosis, kidney and gall stones, and tooth decay are associated with excess acidity in the body.
"... pH paper test using saliva represents the most consistent and most definitive physical sign of the ionic calcium deficiency syndrome ... The saliva pH of the non-deficient and healthy person is in the 7.5 to 7.1 slightly alkaline range. The range from 6.5 which is weakly acidic to 4.5 which is strongly acidic represents states from mildly deficient to strongly deficient, respectively. Most children are dark blue, a pH of 7.5. Over half of adults are green-yellow, a pH of 6.5 or lower, reflecting the calcium deficiency of aging and lifestyle defects. Cancer patients are usually a bright yellow, a pH of 4.5, especially when terminal."...The Calcium Factor: The Scientific Secret of Health and Youth, Carl J. Reich, M.D., Gilliland Printing Inc., Arkansas City, Kansas, 1996.
"PH paper strips to measure acid/alkaline pH balance belong in every family medicine kit, right beside the thermometer and bandages." - Dr. R. Dunne
The blood regulates itself by depositing and withdrawing acid and alkaline minerals from other locations including the bones, soft tissues, other body fluids and saliva. The pH of these other tissues and fluids can fluctuate greatly in an unhealthy, acidic body, but will be just a little bit less alkaline in a healthy, balanced body. The pH of saliva offers a window through which you can see the overall pH balance in your body.
Here are some general guidelines for judging your saliva pH test results:
- A young, healthy child will read at 7.0 - 7.5
- A healthy adult will read at 6.8 - 7.4
- An adult may not have any apparent illness at 6.8, but is starting to become deficient.
- Mildly deficient 6.5 [There will probably be some starting signs of illness, allergy, arthritis or disease.]
- Severely deficient and diseased 4.5 [Most likely has cancer.]
Testing Your Urinary pH
The pH of the urine indicates how the body is working to maintain the proper pH of the blood. Urine reveals the alkaline building and acid tearing down cycles. The pH of urine indicates the efforts of the body via the kidneys, adrenals, lungs and gonads to regulate body pH balance through the buffer salts and hormones. Urine can provide a fairly accurate picture of body chemistry, because the kidneys filter out the buffer salts of pH regulation and provide values based on what the body is eliminating. Urine pH can vary from around 4.5 to 9.0 for its extremes, but the ideal range is 6.5 to 7.0. Urinary pH tends to be lower in the morning and higher in the evening.
The pH of the urine can vary widely. The pH of urine is also affected by the biochemicals that the body is eliminating. These include biochemicals such as excess minerals, vitamins, and products of metabolism and also include drugs and toxins being eliminated by the body.
The pH of the urine is not as affected by digestive enzymes as salivary pH. However, the pH of the urine can be affected by:
- pollutants you breathe
- preservatives you eat
- stress you encounter
- the food you eat
- how much water you drink
- the amount of pathogens in your system
- how much rest you receive
- all the biochemical activities going on in your body.
What do you do if your body is too acidic?
Generally it is best to consume 80% of our diets as alkaline and 20% acidic. Be careful with sodium though. An excess of sodium is associated with a multitude of diseases, such as cancer, arthritis, and osteoporosis. See work of Dr. Gerson.
A Brief Science of Body pH
To determine if a food is acid or alkaline, it is burned and the ash is mixed with water. If the solution is acid or alkaline then the food is called acid or alkaline. Ash is the mineral content of the food. Acid minerals include: chlorine (Cl-), sulfur (S-), phosphorus (P-), and they form hydrochloric acid (HCl), sulfuric acid (H2SO4), and phosphoric acid (H3PO4).
Minerals with a negative electrical charge are attracted to the H+ ion. These are called acid minerals. Acid minerals include: chlorine (Cl-), sulfur (S-), phosphorus (P-), and they form hydrochloric acid (HCl), sulfuric acid (H2SO4), and phosphoric acid (H3PO4).
Minerals with a positive electrical charge are attracted to the negatively charged OH- ion. These are called alkaline minerals. Nutritionally important alkaline minerals include calcium (Ca+), potassium (K+), magnesium (Mg+), and sodium (Na+). (Cancer patients tend to have an excess of sodium. - Gerson page 97).
To determine if a food is acid or alkaline, it is burned and the ash is mixed with water. If the solution is acid or alkaline then the food is called acid or alkaline. Ash is the mineral content of the food.
Also, while it is commonly understood that the body needs calcium to build bones, what is not generally known is bones are a complex matrix of many different minerals and if all the required minerals are not present then strong bones cannot be built. There are at least 18 key bone-building nutrients essential for optimum bone health. The implication is that it is easier to destroy bone through excess acidity in the body than it is to rebuild bone.
Furthermore, as farm soils become depleted of many trace minerals the foods grown on these soils contain less and less of the required nutrients. At last count, the human body requires 90 different nutrients for optimum health, and the list is growing year by year
How does this relate to body metabolism?
Basically, if the body fluids are acid they will seek alkaline minerals to react with - such as sodium, potassium, zinc, iron, calcium. These are found in the liver, muscles, ligaments and bones, etc., if too little is available from the diet. But why should this happen? Effectively, all the body's internal fluids are designed to be slightly alkaline, such as interstitial fluid, cerebrospinal and lymphatic fluid, liver bile and so on. The only exception to this is the hydrochloric acid produced by the stomach.
While our bodies are designed to be alkaline, cells produce acid as a by-product of their normal activity. The acid waste matter thus produced is reduced to carbon dioxide and water which are excreted harmlessly from the body.
| Examples of Common Food Types that have a Strongly Acid pH(avoid) | Examples of Common Food Types that are Mildly Acidic | Examples of Common Food Types that are Mildly Alkaline | Examples of Common Food Types that are Strongly Alkaline(Best) |
| red meat | grains | tofu | soy |
| alcohol | legumes | vegetables | vegetables |
| eggs | most nuts | olive oil | real salt |
| dried fruit | canola oil | goat milk | sprouts |
| sugars | fruit juice | almonds | garlic |
| hard cheese | milk, rice/ soy milk | font face="Times New Roman">buckwheat | alkaline water |